EDdy Dynamics,
mIxing, Export, and Species composition
(EDDIES)
Project Summary and Cruise Logistics
Updated April 7, 2004
Contents:
Part 1 – Project Summary
1. Hypotheses
2. Scenarios
3. Objectives
4. Project timeline
5. Related projects and proposals
Part 2 – Cruise Planning
6. Selection of the target eddy feature
7. Sampling operations
8. Measurements
9. Water budget
10. Personnel and watch schedule
11. Lab space
12. Bermuda port stop logistics
Appendices
A. Detailed sampling information: Café Thorium
B. Detailed sampling information: Siegel
C. Detailed sampling information: Hansell
D. Tracer cruise logistics: Ledwell
E. Detailed sampling information: Falkowski
F. Detailed sampling information: Bates
G. Detailed sampling information: Steinberg
1. Hypotheses
Prior results have documented eddy-driven transport of nutrients into the euphotic zone and the associated accumulation of chlorophyll. However, several key aspects of mesoscale upwelling events remain unresolved by the extant database, including: (1) phytoplankton physiological response, (2) changes in community structure, (3) impact on export out of the euphotic zone, (4) rates of mixing between the surface mixed layer and the base of the euphotic zone, and (5) implications for biogeochemistry and differential cycling of carbon and associated bioactive elements. This leads to the following hypotheses concerning the complex, non-linear biological regulation of elemental cycling in the ocean:
H1: Eddy-induced upwelling, in combination with diapycnal mixing in the upper ocean, introduces new nutrients into the euphotic zone.
H2: The increase in inorganic nutrients stimulates a physiological response within the phytoplankton community.
H3: Differing physiological responses of the various species bring about a shift in community structure.
H4: Changes in community structure lead to increases in export from, and changes in biogeochemical cycling within, the upper ocean.
2. Scenarios
There are several scenarios in which this chain of hypotheses could be linked or broken. These include, but are not necessarily limited to, the following:
S1: Nutrient input to the euphotic zone simply increases the rate of production by the background species assemblage dominated by picoplankton; impacts on biogeochemical cycling are nil.
S2: Increased nitrate availability stimulates a bloom of diatoms; silica-rich organic material produced in the bloom sinks rapidly out of the euphotic zone once the nutrients are exhausted.
S3: Shoaling isopycnals transport DIP closer to the surface, facilitating nitrogen fixation by Trichodesmium or perhaps vertically migrating diatoms with symbiotic bacteria; nitrogen-rich organic material produced during the bloom is exported primarily in dissolved form.
S4: The eddy feature accommodates a change in community structure and biomass of consumers that produce rapidly sinking particles.
3. Objectives
The following objectives are designed to test hypotheses H1-H4 and distinguish between the scenarios S1-S4 in which the chain of hypotheses are linked or broken.
O1: Measure the enhancement of inorganic nutrient availability brought about by eddy-induced upwelling.
O2: Measure the phytoplankton physiological response to increased nutrients.
O3: Assess shifts in species composition associated with the eddy disturbance.
O4: Quantify the impact of the eddy disturbance on upper ocean biogeochemical cycling: measure elemental inventories, primary production, and export.
O5: Assess the interaction between eddy-driven isopycnal transport processes and diapycnal fluxes in and below the mixed layer (quantifies the reversibility of the eddy-induced transport; specifically, how is the oxygen anomaly “left behind” and eddy-driven new production event).
4. Project timeline
2003
July 1 Start Date
July 23 First PI meeting, Woods Hole
2004
February Second PI meeting, ASLO/TOS Conference, Honolulu
Summer Field Work
2005
Winter PI meeting: analysis of ’04 and planning for ‘05
Summer Field Work
2006
Winter PI meeting: synthesis and manuscript preparation
June 30 End date
5. Related Projects and Proposals
Participation starting in 2004:
Steinberg Zooplankton net tows
Davis VPR
Carlson prokaryotic community structure and DOM dynamics
Armbrust genetic diversity in eukaryotes
Oakey/Ledwell Finestructure
Participating in 2005:
Shipe/Brzezinski N and Si uptake
Fratantoni Gliders
Schofield Gliders
Benitez-Nelson et al. Hawaiian Eddy Project “EFLUX”
http://www.soest.hawaii.edu/oceanography/eddy/
6. Selection of the target eddy feature
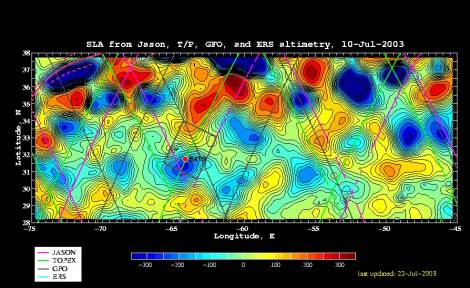
Real-time analysis of altimetric observations will provide maps of the eddy field prior to and during our sampling operations (Figure 1).
|
|
|
Figure 1: Example objective analysis of sea level anomaly (SLA) for 10 July 2003. Near real-time data feeds from Jason, Topex, Geosat Follow On, and ERS were obtained through the Colorado Center for Astrodynamics Research Real-Time Altimetry Project; see http://ccarwww.colorado.edu/~realtime/welcome/. |
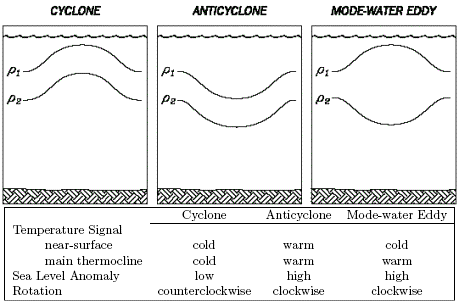
As far as we know, there are three different types of mid-ocean eddies in the Sargasso Sea (Figure 2): cyclones, anticyclones, and mode-water eddies (MWEs). Cyclones and MWEs are of interest to this project, as both tend to displace upper ocean isopycnals toward the surface, causing nutrient input into the euphotic zone. Whereas cyclones are identifiable in satellite altimetry by virtue of their negative sea level anomaly (SLA), MWEs are not distinguishable from anticyclones on the basis of altimetry alone because both result in positive SLA. In principle, satellite-based SST could distinguish these two, as anticyclone and MWEs would be characterized by warm and cold SST anomalies, respectively. However, given the paucity of reliable SST imagery in the Sargasso Sea during summer, we will likely have to rely on in situ measurements to unequivocally distinguish MWEs from anticyclones.
|
|
|
Figure 2. Three different types of eddies in the Sargasso Sea. |
Eddy age is another key issue. Whereas an intensifying cyclone will have upwelling in its center, the isopycnals in the interior of a decaying cyclone will be downwelling. The earlier phase of the eddy’s lifetime will be when nutrient injection and the associated biological response occur.
Summary of desirable characteristics for the target eddy:
1. Young
2. Strong imprint on upper ocean physics, biology, and chemistry
(not necessarily a large amplitude SLA in the case of a MWE).
3. Intensifying
4. Chemical impact discernible in real time (optical NO3 sensor)
5. Biological impact discernible in real time (fluorometry, microscope counts, VPR?)
6. Cyclone versus MWE?
a. unequivocal satellite determination favors cyclones
b. trapping of near-inertial motions and possible enhanced mixing favors MWEs
c. some of the big events at BATS have been MWEs
Jenkins (1988) Summer 1986 event
McNeil et al. (1999) July 1995 eddy
7. Proximity to BBSR: must be within 1 day’s steam for Weatherbird II
Clearly, it will behoove us to sample several eddies during the first survey cruise prior to making a decision about which eddy we wish to spend the rest of the summer in.
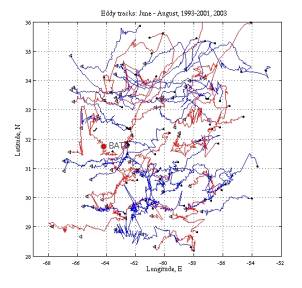
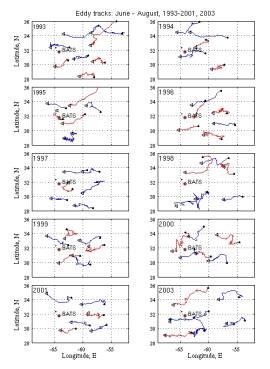
Eddy statistics from prior years can provide some guidance as to what to expect:
|
|
|
|
Figure 3. Eddy tracks for June through August, 1993-2001, 2003. Cyclones are in blue, and anticyclones are in red.. |
|
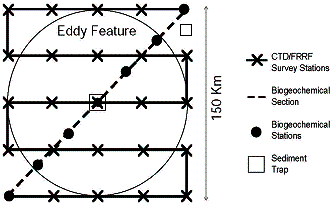
7. Sampling operations
Once the target feature is chosen, intensive sampling will begin:
Oceanus: 2 snapshots per “Survey Cruise”
Weatherbird II: 2 sections per “Section Cruise”
Timeline:
6/11
– 7/3 7/6 – 7/22 7/25 – 8/11 8/14 – 8/29
Oceanus Survey 1 Tracer 1 Survey 2 Tracer 2
Weatherbird II Sections 1 Sections 2
Note that five days of ship time (originally requested for a test cruise in April) have been added to the first survey cruise. This shifts the timing of all subsequent cruises from an earlier version of the schedule.
Oceanus
Survey Timeline (23 days total)
Transit
3.5 days outbound (includes test stations in slope water, Gulf Stream, and Sargasso)
1.5 days inbound
Search: 5 days
VPR + XBT underway survey
CTD casts and MOCNESS tows: (1) eddy cores, (2) eddy edges, (3) “ambient”
First snapshot: 6 days
CTD grid with noon and midnight MOCNESS tows – 4 days
25 stations at 1 hour per station
½ hour bio-optical casts for stations falling between 0900-1600 hrs
8 MOCNESS tows at 3 hours per tow
2 days steaming time
VPR survey – 2 days
Second snapshot: repeat of first snapshot, 6 days
Joint operation with Weatherbird (intercalibration stations)
Weatherbird II
Survey Timeline (10 days total)
Day 1: Transit to Eddy Center (EC)
Day 2: Deploy Production array at EC
Perform repeated profiling at EC
Deploy TRAPS at EC
Recover PP array
Day 3: Transit to non-eddy waters
Deploy control TRAPS
Transit to Station #1 (SW corner)
Start synoptic transect survey
(SW to NE)
Day 4: Underway surveying
Day 5: Complete Transect (stations 1-7 , SW to NE)
Resample stations towards EC
Day 6: Production deployment at EC
Repeated profiling at EC
Recover Production array
Recover TRAPS deployed at EC
Day 7: Resample stations 3,2 &1
Day 8: Recover control Traps
Day 9: Proceed to BBSR
Day 10: Arrive back at BBSR
We would get 3 biogeochemical hits at the EC with repeated profiling during the 2 primary production days. We would get 2 traps deployments (EC & control) of about 4-5 days duration. I have just made rough estimates of on-station and underway time but above should be fine. If we wanted to finish at the EC (4th hit) then we could recover control TRAPS directly after recovering EC-TRAPS then revisit Station #1,2,3 and Finish at 4 (EC). Also, I guess we will need to add some time in case we recover Dave's drifter.
8. Measurements
Remote sensing
Altimetry, ocean color, SST McG/Siegel
Oceanus Surveys
XBT McG
ADCP McG
CTD + FRRF + Optical NO3 McG/Falko/Siegel
Niskin water sampling Hydro team
Turner Chl Siegel
HPLC Siegel/McG
Flow cytometry Bates
NO3, PO4, SiO2 Siegel/McG
POC, PON Bates
Helium/Tritium Jenkins
Microscope counts Falko
Bio-optics (casts) Siegel
Bio-optical drifter Siegel
VPR Davis
Weatherbird II Sections
NO3, PO4, SiO2; HPLC; POC, PON Bates
14C Productivity Bates
Sediment traps (150m) Bates
TCO2, pCO2, O2 Bates
DOC, DON, DOP, δ15N(PON) Hansell
Thorium-based export Buesseler 2hr casts edge/middle/center
Oceanus Tracer Cruises
SF6 Ledwell ship available half time
Gliders
CTD, fluor., opt. backscatter, PAR Fratantoni
Notes:
A “control” sediment trap will be placed outside the eddy. BATS will also serve as a control.
FRRF work at night; can dark adapt (1 hour delay)
Falkowski may bring along a FlowCAM
Niskin sampling depths:
Prior work used 0,20,40,60,80,100,120,140,200,300,500,700 (12 depths)
Should we increase resolution near the base of the euphotic zone?
Bottle sample inventory
Oceanus: (2 cruises x 2 snapshots x 25 stations + 15 search) x 15 depths = 1725 samples
HPLC: down to 140m only (11 depths) = 1265
Weatherbird II: 2 cruises x 2 sections x 7 stations x 15 depths = 420 samples
HPLC: down to 140m only (11 depths) = 308 samples
Utilizing down time on Ledwell cruises
Sampling sled stays attached to hydro wire
Sample 4-5 hours; process 4-5 hours [ship available during processing]
Note: no CTDs possible prior to injection on Tracer 1.
Oceanus salt bottles to be stored aboard ship and run upon return to WHOI; experience suggest storage period of up to one year is acceptable, provided that bottle neck and cap are dried thoroughly prior to closure.
Samples will be collected for flow cytometric analysis; BATS currently has these run by the Chisholm laboratory at $15/sample.
BBSR to supply Winkler system plus backup for Oceanus.
Logistics:
Ledwell van shipped to Bermuda
McG et al. sail with 20’ storage van aft stbd
Swap vans between Survey 1 and Tracer 1
Swap vans between Tracer 1 and Survey 2
Swap vans between Survey 2 and Tracer 2
McG et al. van shipped from Bermuda to WHOI
9. Water budget
Oceanus
Salt bottles 0.25 l
NO3, PO4, SiO2 0.06 l
O2 0.14 l
Chl 0.50 l
HPLC 4.00 l
Flow cytometry 0.02 l
POC, PON 4.00 l
TCO2, pCO2, Alk 2.00 l
DOC, DON, DOP 0.40 l
FRRF,Microscopy 0.20 l
Helium flux gauge 0.50 l
Total 12.07 l*
*Note does not include water for rinsing. Falkowski group also requires 1l from the surface and chlorophyll maximum for phytoplankton counts.
del 15N (PON) bucket samples
Sampling depths (12): 0,20,40,60,80,100,120,140,200,300,500,700
Sampling depths (15): above plus 50,70,90 [10m resolution 40-100m]
[using 2nd bottles from 200,300,500,(700)]
10. Personnel and watch schedule
Oceanus Survey Cruises [capacity 18]
McGillicuddy 3-4 *
Bates 2-3 *
Hansell 1 *
Falkowski 3
Siegel 1
Jenkins 2
Steinberg 2
Davis 2 [+1]
*Hydro team will consist of 8 people total, two 4-person shifts (12 hours on, 12 hours off). This approach was decided upon at the Honolulu meeting from three potential solutions:
Option 1:
6 people total, two 3-person shifts (12 hours on, 12 hours off)
-very short on manpower
-can be covered by McG/Bates/Hansell personnel
Option 2:
8 people total, two 4-person shifts (12 hours on, 12 hours off)
-best in terms of manpower
-will require borrowing 1 person from another group
Option 3:
9 people total, three 3-person shifts (4 hours on, 8 hours off)
-short on manpower, but off-watch personnel fill in at times
-will require borrowing 2 people from other groups
Oceanus Tracer Cruises [capacity 14-18]
Ledwell 6
Weatherbird II [capacity 9]
Bates 3*
Hansell 1*
Buesseler 2
Carlson 1-2 #
Armbrust 1-2 #
* These 4 people comprise the hydro team that will run 12 on / 12 off shifts.
# Carlson and Armbrust will have a total of three berths between them.
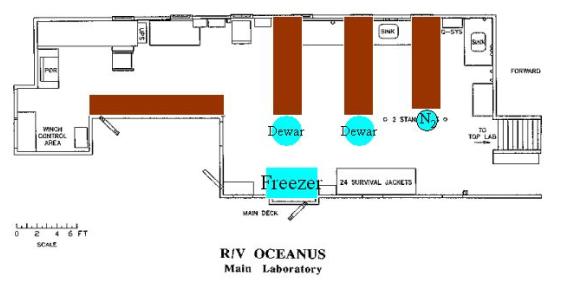
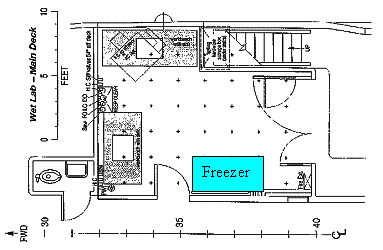
11. Laboratory Space
Space needs:
Jenkins 15’, sink McG 10’ Bates 12’
Falkowski 10’ Steinberg 7’, sink Hansell 5’
Siegel 4’ Davis 8’ aft Hydro 24’ Total: 93
Space available:
Main lab bench: 67’
Wet lab bench:
Jenkins van:
Layouts:
12. Bermuda port stop logistics
11 June: Survey 1 departs WHOI
3 July: Survey 1 arrives Bermuda, pier available upon arrival
5 July: Ship moves to anchorage at 0700 hours
6 July: Tracer 1 departs Bermuda
22 July: Tracer 1 arrives Bermuda, pier available at 1530 hours
25 July: Survey 2 departs Bermuda
11 August: Survey 2 arrives Bermuda, goes straight to anchorage
12 August: Ship moves from anchorage to pier at 1530
14 August: Tracer 2 departs Bermuda
29 August: Tracer 2 arrives WHOI.
Prior correspondence:
Port Stop 02 July - 05 July
Pier Available - Arrival on 02 July
through 0700 hrs. 05 July
Port Stop 20 July - 23 July
Pier Available - 1530 hrs. 22 July through
departure on 23 July
Port Stop 09 August - 12 August (updated 2/10/04)
Monday to Thursday 09/12 August, we could get you alongside
Ordnance Island Dock from arrival to 1100/Tuesday and then again from
1530 Thursday until sailing (This now has changed, no berth available until
1530/12 August)
Only option would be to anchor in St. George's Harbour or re-schedule and
berth 1530/12 August and remain in until that week end stop.
All other time would be spent at anchor.
BBSR has a 42 ft boat the R/V Stommel that could be used to assist during our
port stops. The cost is $325 per half day. They do get booked up and may only
be able to assist us during lunchtime and after 5pm. There are water taxis
available for people. Captain Bearse has also used the ship's zodiac for people
in the past. That would allow us to use the limited pier time to load unload
any large equipment.
Appendix A: Detailed sampling information - Café Thorium
4 Liter sampling plan
- Approximate number of 4 liter samples: 120-140/cruise
This plan is based on 10-12 point profiles collected at each station.
- 7 stations to be sampled during BGC section 1 will include: 2 out stations, 2 edge stations, 2 middle stations, 1 center station. 6 stations sampled on BGC section 2 include: 1 out station (excluding the most recent out station), 2 edge stations, 2 middle stations, 1 center station.
- 1 deep water (4000 meter) calibration cast is required. 10 samples for 234Th calibration collected.
- We estimate a 4l sample collection rate of 20/day.
- Approximately 3 days are needed for sample processing. Will require 2 days at the dock to finish processing.
- All beta counting will be performed back at WHOI.
- In ships lab, rack space for filtration apparatus will require 4x8 feet of wall space. (We are requesting the aft section of the WB II main science lab. See deck layout)
- Berthing requirements: 2 persons on each leg. (Need to find 1 person for June ’04 and July “04 cruise)
- Jan ’05: recovery chemistry at WHOI (Need 1 person for lab work: Claudia student?, Pinghe Cai?)
Pump sampling plan
- A total of 8 stations sampled BCG Sections 1 and 2. Section 1: 2 out stations, 2 edge stations, 1 eddy center. Section 2: 1 eddy center, 1 edge, 1 out
This plan is based on 4 point profiles collected at each station.
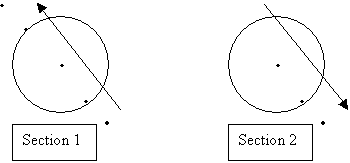
- Casts will consist of 4 pumps deployed simultaneously for 2 hours, or 2 casts with 2 pumps deployed for a total of 4 hours. Total volume of 500l at 8 l/min. 1 hour total deployment/recovery time.
- Wire time required: 16 hours w/4 pumps, 32 hours w/2 pumps.
A request has been made to have 4 pumps available so as to minimize wire time requirements.
· Pump deployment will require separate hydro-wire and winch.
· Each pump will be fitted with a 142 mm 54um screen followed by a 1.0 um quartz filter. Total of 64 particulate samples for C/Th analysis and counting. (32 silver filters/cruise)
Other:
· Will there be a drogued PITS traps deployed by BATS team?
Should a trap be shipped back from Hawaii for the July ’04 cruise?
· Counting: 54 um samples need to be counted first. (Typical BATS activity for these samples is as low as 0.6 cpm) 3 days counting time required for all silver filters.
· 14 days required for counting 4l samples. All counting finished in 14-16 days from collection date. (BATS samples are around 3 cpm after 10 days)
Appendix B: Detailed sampling information – Siegel
The UCSB group will make three field measurements in support of EDDIES 2004.
1. Spar array between the two survey cruises
2. Profiling spectroradiometery casts to characterize UW light field in and around the eddy from the survey cruises
3. Autonomous NO3 sensor on the survey CTD package
The spar array will be made up of 7 SBE-39s (temperature loggers with 2 millidegree accuracy) and 3 WETLabs chl-fl/backscatter sensors (ECO-FL-NTUSB; http://www.wetlabs.com/Products/eco/flntu.htm). There may be more instrumentation deployed depending on budgets, etc. The spar will have a surface light and an ARGOS transmitter (maybe two) and will be drouged with a holey sock at the depth of the tracer release. Dave Menzies can handle this and will be on both cruises; modulo some help on deck deploying and retrieving.
The profiling spectroradiometer is a Satlantic SPMR system with 12 or so channels of downwelling irradiance, upwelling radiance, chl-fl and temperature on the fish and a surface sensor for measuring incident downwelling irradiance. The SPMR is a hand-lowered instrument that is designed to minimize ship shadows. There will be a small hand reel for the cable that needs to be bolted to the deck. We want casts from daylight hours (preferably 0900 to 1600) and normally deploy from the stern with stern facing the sun. The deployment takes two people minimally; one running the aquisition computer and one handling the kevlar cable. With Dave M going alone from UCSB, we will need some help with this (either on deck or in the lab). The deployments take less than 20 minutes per station. The surface sensor needs have an unobstructed view of the sun, be able to run cables into the lab computer and have a seawater supply via a garden hose (for cooling). We've done this on the Ron Brown before and will on the Knorr this fall. The computer should be near access to the fantail and we may want to use radios to communicate from lab to deck.
The last is that we'll be responsible for the care and feeding of the optics sensors on the survey CTD and the UCSB NO3 optical nitrate sensor. This should be easy but will require us to do air cals of the transmissometers, etc.
I figure we will need 3 to 4 feet of lab space for all of this although a good deal of storage for the spar equipment. I'm not sure of hazmats at this time, but I think we'll have ethanol for cleaning optical windows.
Appendix C: Detailed sampling information – Hansell
Water budget:
DOC 100 ml
DON 100 ml
DOP 200 ml
Del 15N PON (4 L, but only at the shallowest depths). Perhaps we could take some of this with bucket casts, or perhaps these samples should come from the survey.
Appendix D: Tracer cruise logistics – Ledwell
Injection/Initial Sampling Cruise
R/V Oceanus, Ledwell, Chief Scientist
5 July 2004 – 20 July 2004, Bermuda to Bermuda
15 days actually at sea
Day Activity
1 Steam to site
2 Arrive at site, start XBT survey
3 Continue survey, test injection gear, Continue survey
4 Inject tracer, Deploy floats, Deploy Seahorse
5 Let the ship air out, while shifting to sampling mode
6 Sampler Tow 1
7 Sampler Tow 2
8 Sampler Tow 3, Check out Seahorse, redeploy
9 Sampler Tow 4
10 Sampler Tow 5
11 Sampler Tow 6
12 Sampler Tow 7
13 Sampler Tow 8, Turn Seahorse around
14 Steam to port
15 Steam to port
Seahorse is a free-drifting, profiling finestructure instrument from BIO, whose use is not yet funded, but just proposed to NSF P.O. program. If it is funded, it is hard to say how much ship time it will take. It is possible that it will take very little. Each deployment and recovery might take just a few hours.
Days 3 and 4 will be devoted to testing the injector and getting the tracer in. Switching from the injection sled to the CTD/Rosette frame would be impractical during this period. On the other hand we could be finished earlier than planned if all goes well. Injection will be done from the waist of the Oceanus using the starboard CTD boom. On Day 5 we’ll change over to the sampling system – this means shifting the CTD cable to the A-frame on the stern. I believe that we could arrange the termination so that it is easy to switch from the sampler sled to a conventional CTD/Rosette frame. It would be best to do the CTDs from the stern, rather than to move the wire back and forth between the A-Frame and starboard CTD boom, but even this might be arranged with the proper blocks.
Sampling tows
We hang 10 to 12 integrating samplers below the sampling sled, then the sled at the end of the CTD cable, then 10 to 12 more integrating samplers. On the sampling sled is a CTD, a 50-chamber sampling system, with the samplers filling sequentially, and a rosette pylon with a few 1.2 L Niskin bottles. It takes about 90 minutes to deploy (longer the first time). We then get the sled on the target surface, trip the bottles, and tow for 10 hours. Recovery of the system takes another 90 minutes or so. We should allow 14 hours or wire time for the whole operation. We do one of these each day, leaving 10 hours of ship and wire time free for other things. Ordinarily we arrange it so the tow is during the night, e.g. from 2200 to 0800, so the wire is busy from 2000 to 1000. There is nothing sacred about this night time schedule for us. We could tow during the days instead, if someone wanted the wire at night. But it would be hard on us to switch back and forth a lot from days to nights for the tows.
Sampling Cruise
R/V Oceanus, Ledwell, Chief Scientist
12 August to 27 August 2004
Bermuda to Woods Hole (as scheduled presently)
15 days actually at sea.
Once we are at the site and decide where we want to sample, these days are all the same, again with 14-hours of wire time each day to do the sampling tows. All would be the same as described above.
The SeaHorse would be part of the program and would require recovery and redeployment at the start of work and final recovery at the end of work.
The tracer group would use 6 berths, at least, and as presently planned. Extra hands gratefully accepted. The SeaHorse would add 2 people, if funded. But Oceanus has 13 berths and 17 if the berthing van is added.
We need about 60% of the space in the main lab for tracer analysis, CTD and ADCP operations, and data analysis stations. The wet lab will be used for injection gear during the first cruise. We will bring a 20-foot van for the sampling gear for both cruises.
Appendix E: Detailed sampling information – Falkowski
Oceanus: Survey cruises (2-3 berths)
FRRF measurements:
Both continuous and discrete measurements using bench-top-based equipment to generate real-time mapping of variable fluorescence throughout the eddy event. Two machines, each taking up approximately 3 feet of bench space. One will be a single cell FRRF. One will require continuous access to a fresh seawater pump and sink. Discrete samples analysed from water samples from Niskin bottles to generate vertical profiles. A minimum of 100 ml is required per sample; so sampling can be easily combined with other people's experiments. Ideally, 'surface', 'chlorophyll maximum' and 'bottom of euphotic zone' samples are required. Samples taken during daylight need to be incubated in dark/low-light bottles for approximately 30 minutes. In addition, incubation experiments to determine the effect on the FRRF signal of nutrient addition to water samples will be performed, requiring an incubation table on deck with continuous flowing seawater.
Phytoplankton size structure: In addition to the fluorescence measurements, we aim to determine the changes in phytoplankton community and size structure. For this, we filter seawater samples from both surface and depth for microscope analyses and preserve samples for analysis by flow-cytometry at a later date. These measurements will require access to surface water, a filtration rig, and will take up approximately 3-4 feet of bench space. Water requirements will vary depending upon the chlorophyll density in the field.
Oceanus: Tracer cruises (2 berths)
FRRF measurements:
In order to maximize the potential data set obtained during EDDIES 2004, we propose to measure FRRF continuously from the sea surface water during the tracer cruises. This will require a single bench-top FRRF (2-3 feet of bench space) with continuous access to flowing seawater.
Appendix F: Detailed sampling information – Bates
We will have to discuss what the sampling strategy is for both ships but in principle, I would very much like to sample from the Oceanus in the 25 station grid. I would need about 4 m of bench space for DIC and alkalinity analysis on board. I would send the SOMMA (DIC) and VINDTA (TA) system so instead of storing and analyzing samples later (it could be a problem getting that many bottles organized), we'd have a good DIC dataset quickly (I'd replicate sampling of the surface depth for analysis later). I'd need to send at least one person just for the analysis, but hopefully a composite hydro team could share watches, responsibilities for water sampling.
I'm also trying to get an underway seawater pCO2 sensor for the underway water stream for Oceanus. This just needs to be close to the underway seawater line, and tapped off with tygon tubing (no equilibrator, gases, etc.).
Appendix F: Detailed sampling information – Steinberg
a) MOCNESS tows. Tows will be conducted from 0-700 m, with (most likely) intervals of 50 m in the first 200m, then 100 m after that (note- 9 'useable' nets on a MOC-1):
0-50 m
50-100
100-150
150-200
200-300
300-400
400-500
500-600
600-700
It should take about 3 hours actual wire time in water to do each tow- when all goes well. There is additional time needed on deck before and after tow to rig/ take down the net.
We are hoping to get day tows at least 5 of the biogeochemical stations (or along that line), with a paired night tow at min. of 3-4 of them (inside, periphery, and outside the eddy). The optimum time for the day tows is straddling noon, and for the night tows is straddling midnight. (Reasonable windows might be 10 a.m. -3 p.m. for a day tow, and 10 p.m.-3 a.m. for night) The paired tows at each station need to be back to back (i.e., a night tow at a given station will either directly proceed or follow the day tow).
Sample processing is ~8 hrs. per tow if we do everything we'd like to, but time is cut in half with 2 people doing it.
b) If on the Oceanus, we would also like to do some tows in the euphotic zone (with just a regular 1m diameter, 200 um mesh net) at some stations on the radiator grid pattern (as time allows). This may also be helpful in locating the eddy, etc. at the beginning of the first cruise. A 200 m oblique tow takes about 30 minutes wire time. We could certainly do shallower tows (to 100 m or less) and still get interesting info. (Note- the tows we got that preliminary data from were only in the top 10 meters). It's probably a good idea to get replicate tows at a given station, so maybe 2 tows to 100 m is a good compromise. I would base how many stations we do on what time you think is available, and how many samples I think we can handle. Maybe we could handle a third to half of the 25 stations. (I also have to keep being consistent about day or night in mind) But even doing 4-5 stations, covering inside, periphery, and outside is interesting.
An additional thing we can do is 5 minute surface drift tows, which require no wire time, while the ctd is down to do quickie qualitative checks on what's in the water.
c. Sampling scheme- trap samples
We plan to look in the sediment trap samples for counting/ sizing fecal pellets, and qualitative assessment of marine snow, comparing inside and outside the eddy. Usually on the BATS traps there is an "ID sample" that gets saved for just this purpose. But we could use any of the samples, as long as we get to it before it is dried (and also best if preserved in solution, like the BATS "ID samples" are, and not put directly on a filter).
d. Sampling scheme- Acoustics
I have not looked into this at all yet. I don't know that it requires any extra planning right now, as we will use the data that is already being collected on the cruise, but I do need to start investigating what the processing might entail in case there are extra things we need to be aware of.
e. Lab space
We'll need room for a microscope set-up and for sieving/ sorting MOCNESS samples, which will require a sink (hopefully with running seawater hooked up in it). So I'd say ~ 6-7 ft. bench space plus a sink at one end. If there is a lab that has more than one sink in it, so we can have 2 people sieving at the same time, that's even better.
Of course we'd only need the sink/s at certain times, so would be shared with other folks easily.
Will also need some freezer space, a shelf or two, for frozen zooplankton samples.