In order to reconstruct past ocean circulation and climate, scientists measure stable isotopes and trace metals, compiling records that span depth, geography and/or time. They interpret these records to gain insight into size and location of watermasses, or “watermass geometry.” Measurements are typically made on the shells of foraminifera. Foraminifera are very small, ocean-dwelling, unicellular organisms that build calcium carbonate shells. These shells incorporate the chemical characteristics of the water in which they grow. Foraminifera that grow near the sea surface are called "planktonic" foraminifera, and those that live on the seafloor sediment are called "benthic" foraminifera. When foraminifera die, and in the case of planktonic foraminifera, their shells sink to the sea floor, they and other sedimentary particles accumulate in chronological order, the deeper down, the older the shells. By making a series of measurements from different depths on a sediment core, scientists can learn about how the chemistry of the ocean has changed with each accumulated layer of sediment, in other words, over time. Planktonic foraminifera can tell us about changes in surface properties, and benthic foraminifera about changes in deepwater properties.
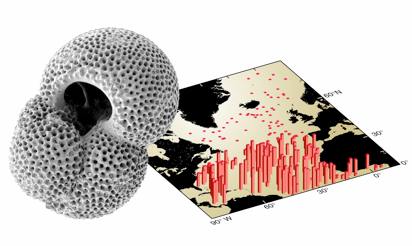
G. ruber, a species of planktonic foraminifera is often used to reconstruct properties of surface water in the tropical oceans (see map of Atlantic distribution above) whereas benthic foraminifera, such as Cibicidoides sp. (below), for example, are often used to infer properties of water just above the seafloor.
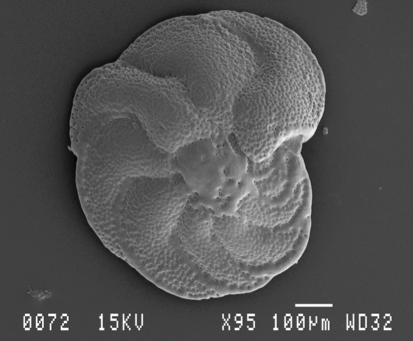
Although there are many proxies we will eventually use to evaluate past changes in ocean circulation once back in the lab, our first approach will be to measure the δ13C of benthic foraminifera from glacial-age sediment. This will enable us to fill the gap in the glacial δ13C transect and determine whether or not Antarctic Intermediate Water crossed the equator during the LGM as it does today.
Isotopes


